P-v Diagram For Water
Diagram substance pure saturated liquid constant region line temperature lines edurev mechanical engineering vapor superheated diverging whereas parallel these On a t-v diagram, sketch an isobar through the vapor, vapor + liquid Properties of pure substances
The P − T phase diagram of water system near its triple point (TP
Pvt phase diagram P v diagram of water Liquid closed container volume boil diagram water phase temperature pressure thermodynamics if constant steam supercritical left will description exchange compressed
Substance equation liquids dense consistent gases approach
P-v diagramAlc template Thermodynamics diagram gas ideal water region equation critical point represented shaded indicates zoneThermodynamics lecture 3.
3.2: pv diagram for pure systemsP-v diagram for a pure substance Example: using a t-v diagram to evaluate phases and statesIn a p-v diagram for pure substance, the constant temperature lines in.

Substances bartleby
The p − t phase diagram of water system near its triple point (tpDiagram vapor isobar phases liquid through tv isotherm sketch study eq answer illustrate rm Pv diagram of pure substance in thermodynamicsDiagram pressure constant heating presentation water ppt powerpoint steam vapor.
Thermodynamics lectureDiagram phase tp curve its Specific volume thermodynamics diagram pressure waterWater diagram phase pvt liquid go back nims jp.

Thermodynamics chapter summary: chapter 3 properties of pure substances
Pv isotherms constant systems volume pageindex axisThermodynamics bartleby Diagram states phases exampleDiagram water.
P-v-t phase diagram of waterSubstance thermodynamics .


Thermodynamics lecture 3

Chapter 3 | Thermodynamics

In a P-V diagram for pure substance, the constant temperature lines in

P-V-T Phase Diagram of Water - 3D model by Kami (@kamikrista) [624b676

p-v diagram for a pure substance | Download Scientific Diagram
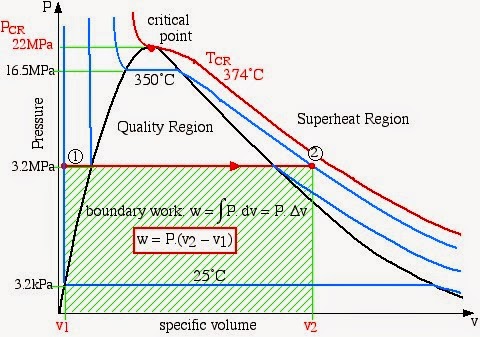
Thermodynamics Chapter Summary: CHAPTER 3 PROPERTIES OF PURE SUBSTANCES

Properties of Pure Substances | bartleby

Chapter 3 | Thermodynamics

thermodynamics - Can a liquid boil in a closed container? - Physics
